
Basic principle of spectrophotometer
The theoretical basis of spectrophotometer is based on the law of light absorption. The law of light absorption is also known as Lambert Beer law.
It is to use the phenomenon of selective absorption of substances under the irradiation and excitation of light to conduct qualitative and quantitative analysis of substances.
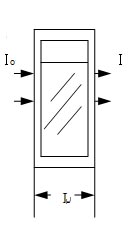
I0:Incident monochromatic light intensity
T =I/I0×100% I:Intensity of emitted monochromatic light
T:Transmittance intensity (transmittance), expressed as a percentage.
Lambert-Beer Law:A=KCL A=log 1/T = -log T
A:Absorbance (specific absorption intensity)
K:Absorption coefficient
C:Sample concentration(g/l)
L:Width of cuvette(cm)
Absorbance:When a beam of parallel monochromatic light is vertically irradiated on a uniform measured sample, the sample will have a certain absorption. The degree to which the sample weakens the absorption of incident light is called absorbance, which can be measured by the instrument. Absorbance is a quantity without unit. It can be seen from the above formula that when the incident light intensity is constant, the absorbance of the substance is directly proportional to the product of the concentration of the light absorbing substance in the substance and the thickness of the substance.
▪ Spectrophotometer of Basic Working Principle2021-10-30More
▪ Basic principle of spectrophotometer2021-10-30More