
Along with the development and progress of modern science and technology, the test means and methods of modern spectrophotometry receives consistent improvement. However, the most basic principle is still based on the Brown-Bill law.
A=KLC
where:
A: refers to value of light absorption needed by an object to be measured at a given wavelength.
K: refers to a factor, called absorption factor of a solution (which is related to The wavelength of light in and the
characteristics of an object to be measured).
L: refers to thickness of an object to be measured (which is often related to the thickness of a cell chamber).
C: refers to the concentration of an object to be measured.
From the above equation, one can see that the absorption of monochromatic light by the object to be measured is proportional to the concentration of the object to be measured.
In practical measurement, monochromatic light reaches photo-electrical receiver through an object to be measured and changed into optical current by photo-electrical amplifier tube or photo-cell. The strong or weak of the light current decides the large or small of the
absorption A .
Assuming light current going through the reference sample is IO, while going through sample to be measured is I and the ratio of the two is T , which is called transmittance rate and expressed in T %.
T = I/Io×100%
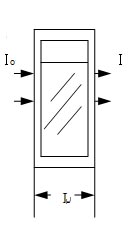
The Brown-Bill Law discovered the change of negative logarithm value of the Transmission ratioratio (which is absorption A) is in proportional to the change of the concentration of the object to be measured.
A= -lg T or lg (IO/I)= KCL
Different substances have different absorption values to the monochromatic light with different
wavelength. This are the characteristics of change that used as the theoretic basis for spectrophotometry
to do qualitative and quantitative analysis on substance.
In view of above mentioned principle, you can know any instrument old or modern, the basic
requirement is to obtain accurate changing feature of the transmission ration of an substance (or sample)
at a given wavelength or absorption within a Given spectrum wavelength range (refer to spectrum characteristic altas).
Double beam spectrophotometer can measure because of which, any influences brought by light source are off-set, so that the accuracy of measurement is ensured from the point of method.
▪ Spectrophotometer of Basic Working Principle2021-10-30More
▪ Basic principle of spectrophotometer2021-10-30More